
Premature ovarian aging (POA) refers to an early decline in ovarian function; it is the main cause of infertility in older women and is characterized by a markedly reduced ovarian reservoir. An interesting review summarized that women born in famine have a significantly earlier menopausal age, which indicates that the neonatal nutrition condition is important to determine follicular reserve and the age of natural menopause. However, the relationship between nutritional conditions during early-life and female reproductive function in adulthood, as well as the specific mechanism, is largely unknown.
Scientists from the State Key Laboratory for Reproductive Medicine of Nanjing Medical University and Reproductive Medicine Center of Nanjing Drum Tower Hospital found that neonatal serum ketone body production could determine the quantity and quality of the primordial follicle pool by reducing ROS-induced primordial follicle apoptosis during follicular reserve formation in the early life. This study is published in Life Metabolism.
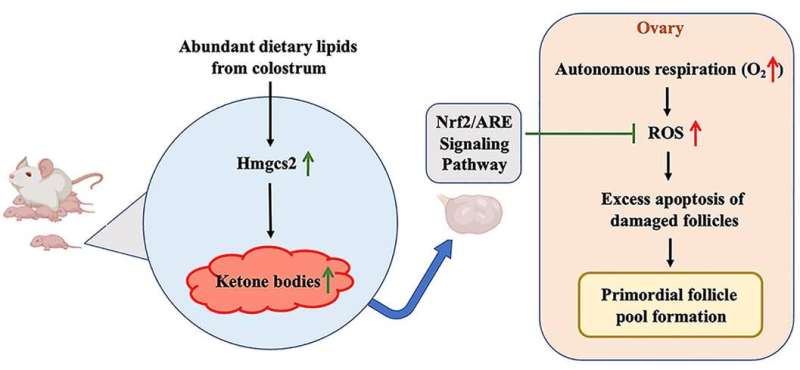
In an animal model, newborns were exposed to rich dietary lipids from colostrum, which transiently stimulate high expression of 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2), the rate-limiting enzyme in most of organs including ovary in neonatal mice. Thus, the neonatal serum ketone body level was significantly higher than adulthood. When Hmgcs2 gene was deleted, the neonatal ketone body level largely decreased, which perfectly mimicked the malnutrition related to ketone body deficiency in newborn mice. Using this ideal animal model, the researchers found that ketone body deficiency in neonatal mice resulted in smaller ovarian follicle reservoir because of the increased apoptosis of primordial follicles, which further led to POA indicated by gradually decreased litter size and prolonged litter interval.
Another challenge the newborns faced was higher oxidative stress because of spontaneous breathing. The ROS production in Hmgcs2 deficient ovary was highly elevated, which led to severe DNA damage of primordial follicle, and thus induced excessive apoptosis of primordial follicles. They further confirmed that β-Hydroxybutyrate (β-HB), supplementation in Hmgcs2 knockout mice could reduce ROS production and alleviate DNA damage induced apoptosis of primordial follicle. Thus, the authors demonstrated that the neonatal ketone body could maintain the quantity and quality of the primordial follicle pool by decreasing ROS production.
Source: Read Full Article